In M600, the new modern building on the green outskirts of Marburg, CSL researches and develops innovative medical therapies for patients with rare diseases. Some of these therapies are plasma-based - i.e. derived from human blood plasma. In addition, there are other biotherapeutics, i.e. drugs that are produced with the help of biotechnological processes.
"To provide treatment options for patients with rare diseases, we work with the latest technologies at the CSL R&D Campus in Marburg and in collaboration with colleagues at other CSL research sites around the world" says Dr. Lars Grönke. He is managing director of CSL's research and development (R&D) in Marburg. This is because CSL also maintains R&D sites in other countries, and usually a drug is not developed at just one of them, but as a joint project involving several sites.
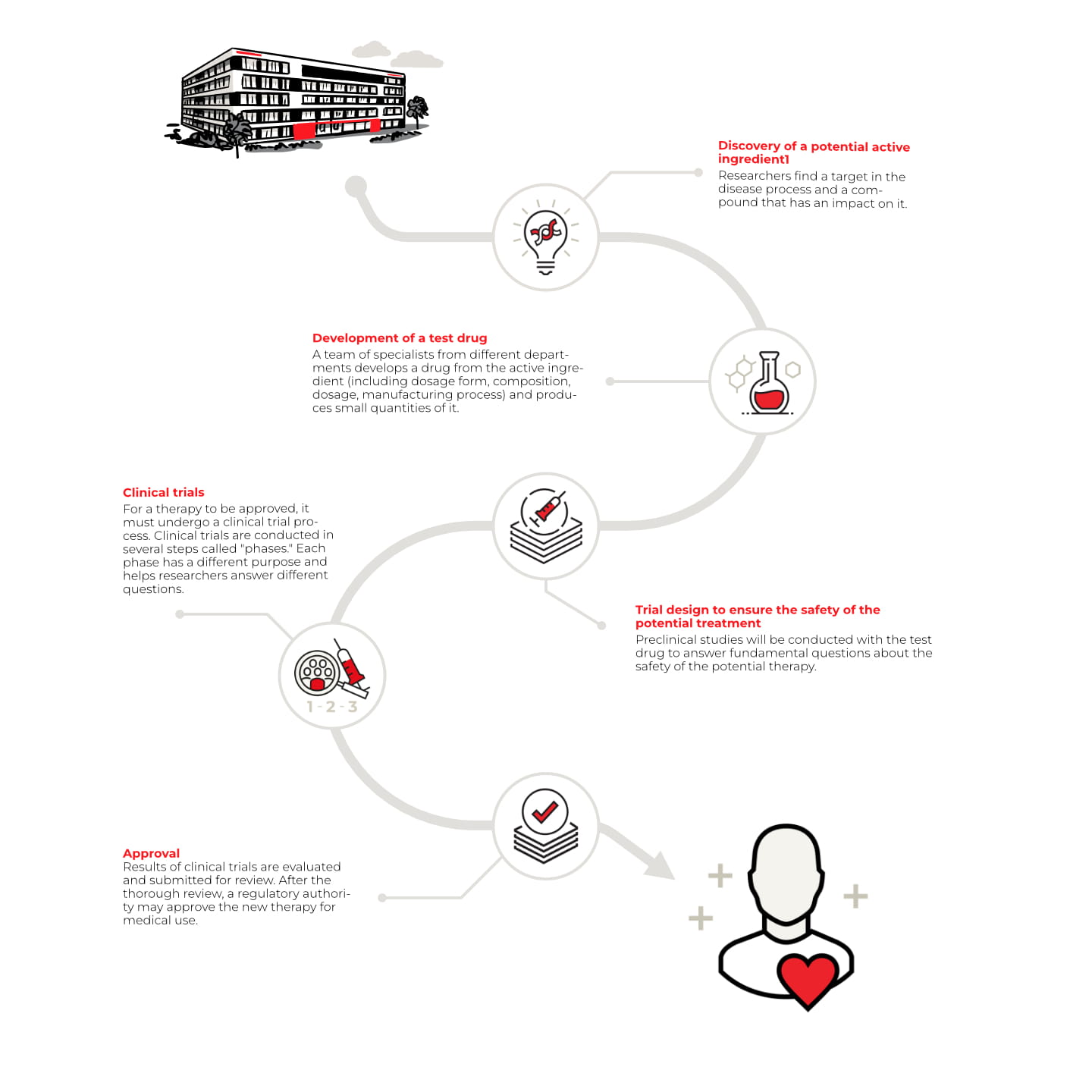
The path of a drug development is extremely complex. On average, it takes 13 years from the idea to the approval of a drug. "This is a path that can only be successfully taken if all those involved work closely together," says Grönke.
"And just as essential are the patients, without whose willingness to participate in clinical trials, no drugs can be developed," says Sylvia Herget, who is responsible for patient partnerships.
" The path of a drug development can only be successfully taken if all those involved work closely together. "
Dr. Lars Grönke, Managing Director, CSL R&D Marburg (CSL Innovation)